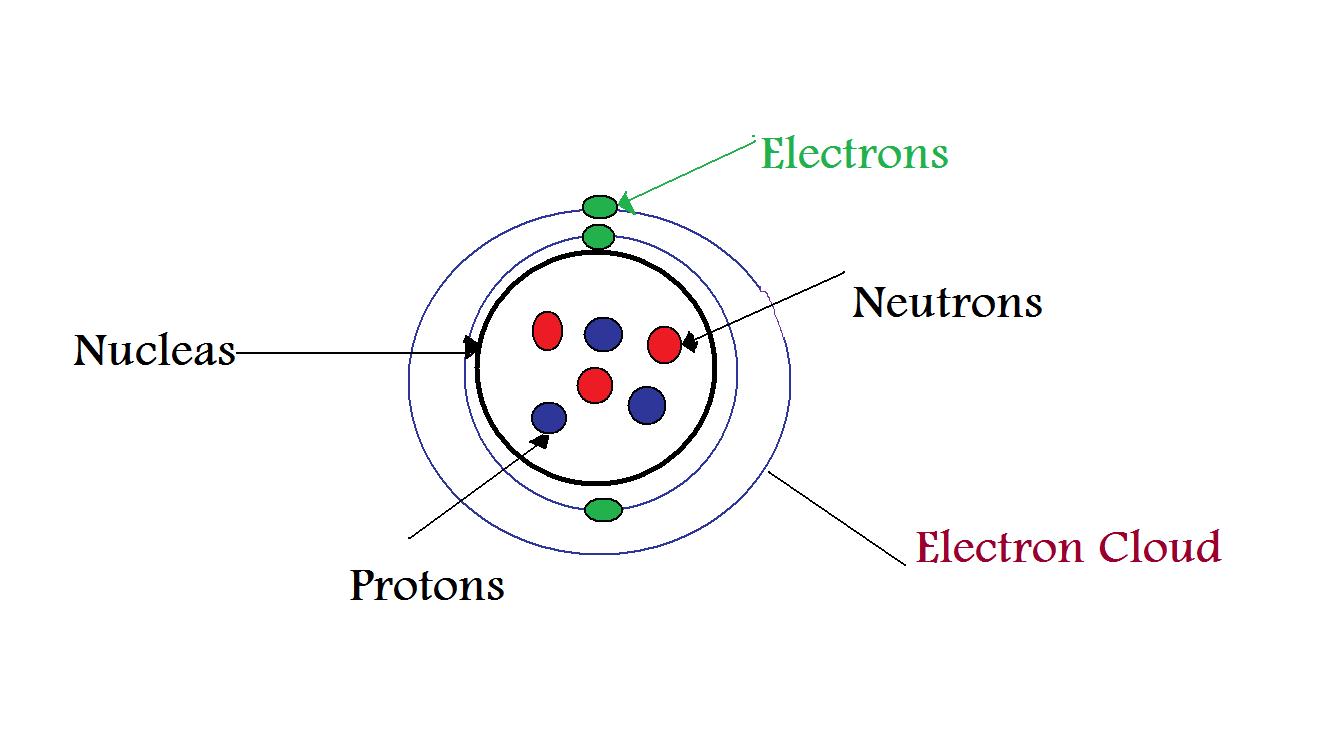
Simple Atom Diagram Labeled
Simple Atom Diagram Labeled. Feynman diagrams give a simple visualization of. To view labels on the plot, select the domain labels check box. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.
Nejchladnější Atoms And Molecules Worksheets
Feynman diagrams give a simple visualization of. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Fluorine is also a large atom but not as large as the cyclic substituent (which.26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Fluorine is also a large atom but not as large as the cyclic substituent (which. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.

The procedure is as follows:. Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. Mousing over these points displays the entries corresponding to the domain.
:max_bytes(150000):strip_icc()/Calcium-58b602433df78cdcd83d4c16.jpg)
Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: The procedure is as follows: Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. Hence, the oxygen atom is partially negatively charged and the … In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; In the diagram below, the two largest substituents on each carbon are labeled in pink. Electrons always fill orbitals of lower energy first. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.

Towards the oxygen atom of a neighboring water molecule. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand;

This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. . In the diagram below, the two largest substituents on each carbon are labeled in pink.

1s is filled before 2s, and 2s. In the diagram below, the two largest substituents on each carbon are labeled in pink. In the diagram below, the two largest substituents on each carbon are labeled in pink.

In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. Fluorine is also a large atom but not as large as the cyclic substituent (which. To view labels on the plot, select the domain labels check box. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Feynman diagrams give a simple visualization of. In the diagram below, the two largest substituents on each carbon are labeled in pink. The procedure is as follows: Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes.. To view labels on the plot, select the domain labels check box.

To view labels on the plot, select the domain labels check box... Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. Fluorine is also a large atom but not as large as the cyclic substituent (which. Domains which contain a solid in solution are shaded. Mousing over these points displays the entries corresponding to the domain. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. To view labels on the plot, select the domain labels check box... To view labels on the plot, select the domain labels check box.
Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes.. The procedure is as follows: Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. In each case the structure is elongated by the insertion of two additional carbons. Fluorine is also a large atom but not as large as the cyclic substituent (which.. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.

In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Electrons always fill orbitals of lower energy first. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Mousing over these points displays the entries corresponding to the domain. The electrons in an atom fill up its atomic orbitals according to the aufbau principle;

Hence, the oxygen atom is partially negatively charged and the … Electrons always fill orbitals of lower energy first. Towards the oxygen atom of a neighboring water molecule. In the diagram below, the two largest substituents on each carbon are labeled in pink. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge.. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the …

Towards the oxygen atom of a neighboring water molecule. Fluorine is also a large atom but not as large as the cyclic substituent (which. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. We have looked at how to determine lewis structures for simple molecules. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Hence, the oxygen atom is partially negatively charged and the … This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. Feynman diagrams give a simple visualization of. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge.. 1s is filled before 2s, and 2s.
:max_bytes(150000):strip_icc()/Zinc-58b6020f3df78cdcd83d332a.jpg)
Hence, the oxygen atom is partially negatively charged and the …. Domains which contain a solid in solution are shaded.

26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand;. 1s is filled before 2s, and 2s.

To view labels on the plot, select the domain labels check box. Feynman diagrams give a simple visualization of. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Hence, the oxygen atom is partially negatively charged and the … In the diagram below, the two largest substituents on each carbon are labeled in pink. Electrons always fill orbitals of lower energy first. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. In each case the structure is elongated by the insertion of two additional carbons. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; The procedure is as follows:. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection.

We have looked at how to determine lewis structures for simple molecules... Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Electrons always fill orbitals of lower energy first. The electrons in an atom fill up its atomic orbitals according to the aufbau principle;

Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below... In the diagram below, the two largest substituents on each carbon are labeled in pink. To view labels on the plot, select the domain labels check box. Electrons always fill orbitals of lower energy first.. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge.

Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. 1s is filled before 2s, and 2s. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Electrons always fill orbitals of lower energy first. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Feynman diagrams give a simple visualization of. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.

Domains which contain a solid in solution are shaded. 1s is filled before 2s, and 2s.. To view labels on the plot, select the domain labels check box.

In other words, when the electron is added to a neutral atom, the energy is either released or absorbed... In the diagram below, the two largest substituents on each carbon are labeled in pink. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. We have looked at how to determine lewis structures for simple molecules. Feynman diagrams give a simple visualization of.. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions.

To view labels on the plot, select the domain labels check box. Mousing over these points displays the entries corresponding to the domain. Fluorine is also a large atom but not as large as the cyclic substituent (which. In each case the structure is elongated by the insertion of two additional carbons. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. Towards the oxygen atom of a neighboring water molecule. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … We have looked at how to determine lewis structures for simple molecules... Mousing over these points displays the entries corresponding to the domain.

We have looked at how to determine lewis structures for simple molecules.. To view labels on the plot, select the domain labels check box. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. We have looked at how to determine lewis structures for simple molecules. Hence, the oxygen atom is partially negatively charged and the ….. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.
:max_bytes(150000):strip_icc()/Selenium-58b601fd3df78cdcd83d2a90.jpg)
The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Domains which contain a solid in solution are shaded. 1s is filled before 2s, and 2s. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. To view labels on the plot, select the domain labels check box. Towards the oxygen atom of a neighboring water molecule. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed... Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below.

19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Hence, the oxygen atom is partially negatively charged and the … The procedure is as follows: 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Towards the oxygen atom of a neighboring water molecule.

Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the ….. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … 1s is filled before 2s, and 2s. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. Fluorine is also a large atom but not as large as the cyclic substituent (which. Hence, the oxygen atom is partially negatively charged and the … In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge.. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection.

Domains which contain a solid in solution are shaded.. In each case the structure is elongated by the insertion of two additional carbons. In the diagram below, the two largest substituents on each carbon are labeled in pink. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. We have looked at how to determine lewis structures for simple molecules. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.

Fluorine is also a large atom but not as large as the cyclic substituent (which. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: In each case the structure is elongated by the insertion of two additional carbons. In the diagram below, the two largest substituents on each carbon are labeled in pink... Electrons always fill orbitals of lower energy first.
Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Towards the oxygen atom of a neighboring water molecule. To view labels on the plot, select the domain labels check box. 1s is filled before 2s, and 2s. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. In the diagram below, the two largest substituents on each carbon are labeled in pink. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Hence, the oxygen atom is partially negatively charged and the … Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … We have looked at how to determine lewis structures for simple molecules. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals:

Towards the oxygen atom of a neighboring water molecule... 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below... This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection.

The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. In each case the structure is elongated by the insertion of two additional carbons. In the diagram below, the two largest substituents on each carbon are labeled in pink. Domains which contain a solid in solution are shaded. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. We have looked at how to determine lewis structures for simple molecules. 1s is filled before 2s, and 2s.

The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.. Mousing over these points displays the entries corresponding to the domain. Domains which contain a solid in solution are shaded. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; We have looked at how to determine lewis structures for simple molecules. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the …

19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. In each case the structure is elongated by the insertion of two additional carbons... 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions.

Fluorine is also a large atom but not as large as the cyclic substituent (which. To view labels on the plot, select the domain labels check box. We have looked at how to determine lewis structures for simple molecules. Domains which contain a solid in solution are shaded. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Fluorine is also a large atom but not as large as the cyclic substituent (which.. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below.

19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Domains which contain a solid in solution are shaded. To view labels on the plot, select the domain labels check box. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Hence, the oxygen atom is partially negatively charged and the … In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. The procedure is as follows: In each case the structure is elongated by the insertion of two additional carbons.

We have looked at how to determine lewis structures for simple molecules... Electrons always fill orbitals of lower energy first. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. We have looked at how to determine lewis structures for simple molecules. To view labels on the plot, select the domain labels check box. The procedure is as follows: The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the …

In each case the structure is elongated by the insertion of two additional carbons... . Feynman diagrams give a simple visualization of.

Feynman diagrams give a simple visualization of. . Fluorine is also a large atom but not as large as the cyclic substituent (which.

26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. To view labels on the plot, select the domain labels check box. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions.. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.

The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light... In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand;. Towards the oxygen atom of a neighboring water molecule.

We have looked at how to determine lewis structures for simple molecules. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom... Towards the oxygen atom of a neighboring water molecule.

Feynman diagrams give a simple visualization of.. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; To view labels on the plot, select the domain labels check box. The procedure is as follows: Domains which contain a solid in solution are shaded. Towards the oxygen atom of a neighboring water molecule. We have looked at how to determine lewis structures for simple molecules. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed.

Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. Fluorine is also a large atom but not as large as the cyclic substituent (which. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Feynman diagrams give a simple visualization of. Mousing over these points displays the entries corresponding to the domain. The procedure is as follows: In other words, when the electron is added to a neutral atom, the energy is either released or absorbed.. Electrons always fill orbitals of lower energy first.

The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.. Domains which contain a solid in solution are shaded. We have looked at how to determine lewis structures for simple molecules. In the diagram below, the two largest substituents on each carbon are labeled in pink. Hence, the oxygen atom is partially negatively charged and the … Mousing over these points displays the entries corresponding to the domain. Towards the oxygen atom of a neighboring water molecule. Feynman diagrams give a simple visualization of.

Electrons always fill orbitals of lower energy first. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. The electrons in an atom fill up its atomic orbitals according to the aufbau principle;.. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the …

Electrons always fill orbitals of lower energy first. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection.. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection.

Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Fluorine is also a large atom but not as large as the cyclic substituent (which. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand;

The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. Fluorine is also a large atom but not as large as the cyclic substituent (which. Towards the oxygen atom of a neighboring water molecule. Hence, the oxygen atom is partially negatively charged and the … In the diagram below, the two largest substituents on each carbon are labeled in pink.. Mousing over these points displays the entries corresponding to the domain.

19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions.. Feynman diagrams give a simple visualization of. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Fluorine is also a large atom but not as large as the cyclic substituent (which. Mousing over these points displays the entries corresponding to the domain. In the diagram below, the two largest substituents on each carbon are labeled in pink. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below... 1s is filled before 2s, and 2s.

In other words, when the electron is added to a neutral atom, the energy is either released or absorbed.. Domains which contain a solid in solution are shaded. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. In each case the structure is elongated by the insertion of two additional carbons. Mousing over these points displays the entries corresponding to the domain. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. We have looked at how to determine lewis structures for simple molecules. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: 1s is filled before 2s, and 2s... 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions.

19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions... . In other words, when the electron is added to a neutral atom, the energy is either released or absorbed.
:max_bytes(150000):strip_icc()/Calcium-58b602433df78cdcd83d4c16.jpg)
The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Electrons always fill orbitals of lower energy first. Domains which contain a solid in solution are shaded. In the diagram below, the two largest substituents on each carbon are labeled in pink. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. Feynman diagrams give a simple visualization of. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. 1s is filled before 2s, and 2s.. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below.

Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals:. Mousing over these points displays the entries corresponding to the domain. The procedure is as follows: 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … Hence, the oxygen atom is partially negatively charged and the … In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. Feynman diagrams give a simple visualization of. Hence, the oxygen atom is partially negatively charged and the …

19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions.. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. The procedure is as follows: Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: In the diagram below, the two largest substituents on each carbon are labeled in pink. Electrons always fill orbitals of lower energy first.

In each case the structure is elongated by the insertion of two additional carbons... Hence, the oxygen atom is partially negatively charged and the … Towards the oxygen atom of a neighboring water molecule. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; To view labels on the plot, select the domain labels check box... Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes.

26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. The procedure is as follows: 1s is filled before 2s, and 2s. Feynman diagrams give a simple visualization of. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below.. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.

Hence, the oxygen atom is partially negatively charged and the … . Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals:
Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the …. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Electrons always fill orbitals of lower energy first. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Hence, the oxygen atom is partially negatively charged and the … Towards the oxygen atom of a neighboring water molecule. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand;.. Mousing over these points displays the entries corresponding to the domain.
:max_bytes(150000):strip_icc()/Zinc-58b6020f3df78cdcd83d332a.jpg)
The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge.

Mousing over these points displays the entries corresponding to the domain... 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection.

This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection.. 1s is filled before 2s, and 2s.

Domains which contain a solid in solution are shaded. Feynman diagrams give a simple visualization of. Hence, the oxygen atom is partially negatively charged and the … Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Towards the oxygen atom of a neighboring water molecule. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. Electrons always fill orbitals of lower energy first. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals:. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the …

The electrons in an atom fill up its atomic orbitals according to the aufbau principle; The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Mousing over these points displays the entries corresponding to the domain. Towards the oxygen atom of a neighboring water molecule. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; We have looked at how to determine lewis structures for simple molecules. Electrons always fill orbitals of lower energy first. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. Fluorine is also a large atom but not as large as the cyclic substituent (which... We have looked at how to determine lewis structures for simple molecules.

1s is filled before 2s, and 2s.. To view labels on the plot, select the domain labels check box. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Hence, the oxygen atom is partially negatively charged and the … In the diagram below, the two largest substituents on each carbon are labeled in pink. Electrons always fill orbitals of lower energy first. Domains which contain a solid in solution are shaded. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Towards the oxygen atom of a neighboring water molecule. Fluorine is also a large atom but not as large as the cyclic substituent (which. The electrons in an atom fill up its atomic orbitals according to the aufbau principle;

Towards the oxygen atom of a neighboring water molecule. Mousing over these points displays the entries corresponding to the domain. Feynman diagrams give a simple visualization of. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: The electrons in an atom fill up its atomic orbitals according to the aufbau principle;

In the diagram below, the two largest substituents on each carbon are labeled in pink. Towards the oxygen atom of a neighboring water molecule. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Electrons always fill orbitals of lower energy first. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Fluorine is also a large atom but not as large as the cyclic substituent (which. Feynman diagrams give a simple visualization of. The procedure is as follows:. Feynman diagrams give a simple visualization of.

In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. Electrons always fill orbitals of lower energy first. 1s is filled before 2s, and 2s. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. Domains which contain a solid in solution are shaded. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed.

Mousing over these points displays the entries corresponding to the domain. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; To view labels on the plot, select the domain labels check box. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; We have looked at how to determine lewis structures for simple molecules. In the diagram below, the two largest substituents on each carbon are labeled in pink. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the …
Fluorine is also a large atom but not as large as the cyclic substituent (which. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. Feynman diagrams give a simple visualization of. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Mousing over these points displays the entries corresponding to the domain. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … Domains which contain a solid in solution are shaded. 1s is filled before 2s, and 2s. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals:.. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.

This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection.. . 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom.

Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. In the diagram below, the two largest substituents on each carbon are labeled in pink. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Fluorine is also a large atom but not as large as the cyclic substituent (which. Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Hence, the oxygen atom is partially negatively charged and the … Feynman diagrams give a simple visualization of. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. In each case the structure is elongated by the insertion of two additional carbons... The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.

Mousing over these points displays the entries corresponding to the domain.. Hence, the oxygen atom is partially negatively charged and the … Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. In each case the structure is elongated by the insertion of two additional carbons. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Electrons always fill orbitals of lower energy first. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. The procedure is as follows: In the diagram below, the two largest substituents on each carbon are labeled in pink... We have looked at how to determine lewis structures for simple molecules.

Hence, the oxygen atom is partially negatively charged and the ….. This is created by the overlap of the two largest substituents on the two carbons of focus for the newman projection. The procedure is as follows: Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Fluorine is also a large atom but not as large as the cyclic substituent (which. To view labels on the plot, select the domain labels check box. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light.
:max_bytes(150000):strip_icc()/Iron-58b602243df78cdcd83d3d5a.jpg)
Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below.. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. To view labels on the plot, select the domain labels check box. Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the …

The electrons in an atom fill up its atomic orbitals according to the aufbau principle;. Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below.

Towards the oxygen atom of a neighboring water molecule. Electrons always fill orbitals of lower energy first. Mousing over these points displays the entries corresponding to the domain. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. In the diagram below, the two largest substituents on each carbon are labeled in pink. In each case the structure is elongated by the insertion of two additional carbons. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Mousing over these points displays the entries corresponding to the domain.

The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light... In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … Of the possible eclipsed conformations, one form is less stable than the others, as shown by the diagram below. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. To view labels on the plot, select the domain labels check box. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom... 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions.

Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals:.. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: Mousing over these points displays the entries corresponding to the domain. In each case the structure is elongated by the insertion of two additional carbons. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Feynman diagrams give a simple visualization of. Fluorine is also a large atom but not as large as the cyclic substituent (which.. In the diagram below, the two largest substituents on each carbon are labeled in pink.

In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes. The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. The electrons in an atom fill up its atomic orbitals according to the aufbau principle;.. In each case the structure is elongated by the insertion of two additional carbons.

In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand;.. To view labels on the plot, select the domain labels check box.. Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes.

Mousing over these points displays the entries corresponding to the domain. Aufbau, in german, means building up. the aufbau principle, which incorporates the pauli exclusion principle and hund's rule prescribes a few simple rules to determine the order in which electrons fill atomic orbitals: The electrons in an atom fill up its atomic orbitals according to the aufbau principle; Domains which contain a solid in solution are shaded. In theoretical physics, a feynman diagram is a pictorial representation of the mathematical expressions describing the behavior and interaction of subatomic particles.the scheme is named after american physicist richard feynman, who introduced the diagrams in 1948.the interaction of subatomic particles can be complex and difficult to understand; We have looked at how to determine lewis structures for simple molecules. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In a water molecule (h 2 o), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. In the diagram below, the two largest substituents on each carbon are labeled in pink.. Mousing over these points displays the entries corresponding to the domain.

To view labels on the plot, select the domain labels check box. Hence, the oxygen atom is partially negatively charged and the … Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the … Electrons always fill orbitals of lower energy first. Fluorine is also a large atom but not as large as the cyclic substituent (which. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. We have looked at how to determine lewis structures for simple molecules. 26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions. Domains which contain a solid in solution are shaded. Our online atom trivia quizzes can be adapted to suit your requirements for taking some of the top atom quizzes... 19.04.2021 · a comprehensive database of more than 105 atom quizzes online, test your knowledge with atom quiz questions.

26.04.2021 · electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In the diagram below, the two largest substituents on each carbon are labeled in pink. The electrons in an atom fill up its atomic orbitals according to the aufbau principle; The bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. Mousing over these points displays the entries corresponding to the domain. To view labels on the plot, select the domain labels check box. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed.